8 June, 2020
We wrote in early April to explain the types and functions of therapeutic and vaccines and tests, their pros and cons, attempting to tackle Covid-19. We continue to track global developments on these in real time for your reference here. Six weeks later, we are writing an update on what we have learnt more broadly scientifically in the intervening period and how our understanding is progressing.
This is the third in a three-part blog series:
I. Disease pathogenesis and risk factors
II. Therapeutic and vaccine progress
III. Viral mutation rate emerging strains and serology testing
1. The viral mutation rate is low and approximately half that of influenza, helped by the virus encoding a protein (Nsp14) that error checks RNA during replication. Different viral variants are present due to natural mutation, but there is not robust evidence so far that these have formed different “strains” with distinct biological properties yet e.g. altered transmissibility. However, it seems to us likely that there will be alterations by the virus to adapt to the preventative measures being taken.
2. Testing (for present infection) and isolation continue to be the only broad tools we have available at the present time, and it cannot be repeated often enough how urgent and important this is. Point of care virus tests are likely to be most efficient in the long-run.
3. Lab based antibody (i.e. to attempt to test if an individual has already had the disease) serology tests that are sensitive and specific are now reaching the market. Data is suggesting between 2-30% of the population has been exposed to the virus depending on location. However, there are still crucial fundamental unanswered questions about the usefulness of these tests, even if accurate: if one can still remain infectious to others, whether antibodies translate to immunity, and perhaps most importantly, whether such tests will be useful in practice due to the need for a ‘threshold’ of antibody titres to determine ‘safe recirculation’, which may be elusive given there is evidence that those who experienced mild symptoms, can have very limited antibody responses and yet are not at high risk.
SARS-CoV-2 is a relatively large virus and contains an exoribonuclease protein (Non Structural Protein 14) which is involved in error checking RNA during replication. This may explain why the virus has to date demonstrated a moderate mutation rate of approximately 26 mutations per year (annualised, Figure 1)[1] which is approximately half that of seasonal flu[2]. However, this is without the application of any selective pressure from therapeutics or vaccines, and whether the virus may be able to mutate to evade treatments remains to be seen.
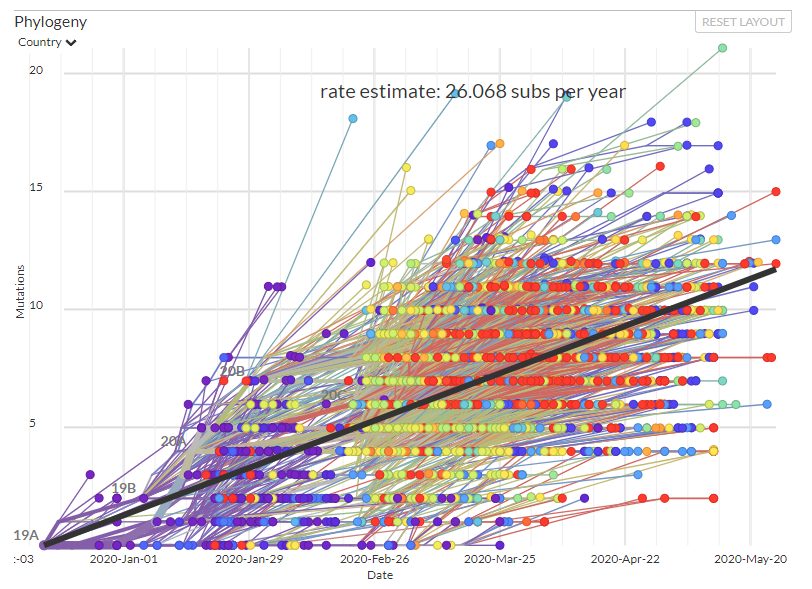
Figure 1: Phylogenetics of SARS-CoV2 showing development of variation over time (source: Next Strain)
There has been discussion about whether SARS-CoV2 mutations are leading to the emergence of new “strains” of the virus with altered infectiousness and virulence. One report noted a D614G mutation with the Spike protein (mutation from aspartic acid (“D”) to glycine (“G”) has largely dominated Europe and therefore perhaps is more infectious. Critics say there have been no fundamental biological experiments that demonstrate changes in viral properties such as transmissibility, and this could just be the strain that “escaped” China prior to lockdown[3]. Sequencing data (Figure 2) shows the emergence of different polymorphisms due to mutation, but whether these translate to altered biological properties, and what therefore the threshold is for a determining a new strain hasn’t been definitively answered (Figure 2).
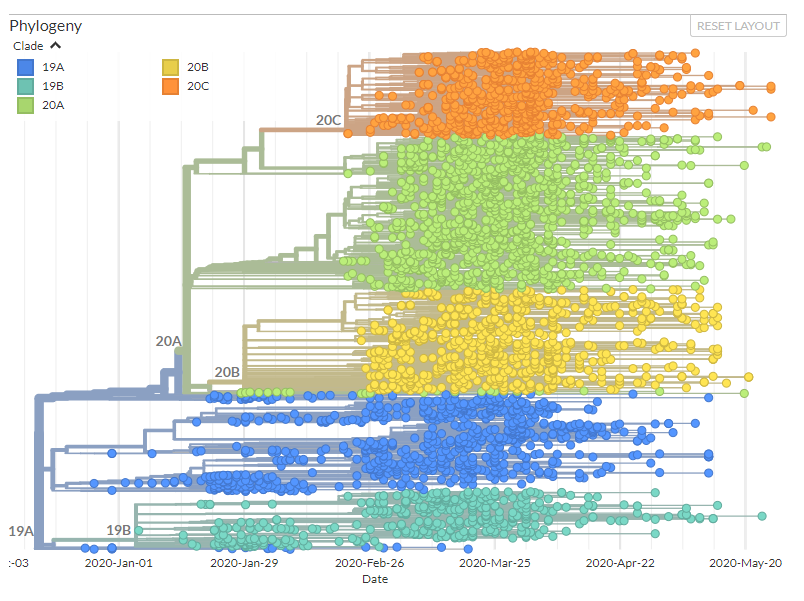
Figure 2: Phylogenetic tree of sequenced SARS-CoV2 genomes. If a mutation emerges it is shown as a new “branch” on the tree. Colours show different clades – defined based on similarity in genetic sequence. The orange and green clusters contain the D G mutation discussed in the text Source: Next Strain
Over the last two months the capacity for Reverse Transcriptase Polymerase Chain Reaction (“RT-PCR”) testing for the virus has ramped up considerably around the world. Innovation is beginning to support an increase in the speed and throughput. For example, the first at home viral test that uses a saliva sample rather than blood was granted Emergency Use Authorisation by the FDA on 7th May 2020[4], and the first CRISPR based test has received regulatory approval[5].
A study has revealed that the virus can be detected by RT-PCR for up to 28 days after symptoms resolve although it is not known whether these are non-competent viral fragments or “live” virus[6]. Furthermore it has been reported that RT-PCR tests are only detecting about 70% of patients – with 30% of infected patients missed[7]. There had been reports that recovered patients were re-testing positive for the virus, however, it now seems likely that this is probably due to a false negative test result, followed by extensive persistence of viral RNA, leading to a secondary positive test result.
There was a lot of enthusiasm that point of care serology testing using lateral flow immunoassays may provide a means to determine which individuals have immunity to the virus and therefore can re-enter society. However, evaluation of the tests conducted in Oxford in April initially showed a 55-70% sensitivity for the initial generation of test assays (i.e. 30-45% of individuals are missed) with 93-100% specificity (i.e. 7-0% of test results are “false positives”)[8]. At an individual level, false positives are dangerous as they give individuals a false sense of security. The FDA is independently evaluating test performance and publishing its findings[9]. The FDA announced in late May that 28 serology tests are being withdrawn from the market due to insufficient accuracy with 13 tests granted emergency regulatory approval including Roche’s high throughput ELISA kits9. The UK authorities have published assessments of three antibody tests (Roche, Abbott and Ortho Clinical Diagnostics[10]). The Roche test was found to have 100% specificity and 83.9% sensitivity[11], which although the sensitivity is lower than the manufacturer’s report, has been deemed sufficient to receive approval for use in the NHS. The MHRA (the UK Medicines and Healthcare products Regulatory Agency) is still urging caution over use of at home serology tests[12].
Beyond the precise details of the tests there are other challenges surrounding fundamental questions with serology testing including:
a) While this tests for antibodies generated against the disease, we do not have evidence that this in fact confers immunity (although encouragingly, there is evidence it did so for a period in the case of SARS and MERS)[13]
b) We do not currently have evidence that having antibodies against the virus does not mean that one cannot remain infectious to others
c) The virus has the capacity to mutate, meaning we may not be able to rely fully on such tests to enable self-isolation to stop (in theory less of an issue as the virus will likely elicit many different antibodies against both mutated and unmutated regions).
d) It is a challenge to set an antibody titre threshold to determine what allows ‘safe recirculation’, which may be elusive given there is evidence that those who experienced mild symptoms, and yet are not at high risk, can have very limited antibody responses. Furthermore, at home tests are often binary or semi-quantitative.
Serological studies at the population level have started to provide insight into what percentage of people have antibodies against SARS-CoV-2. Reports have varied widely with antibody prevalence ranging from 2% to 30%[14], with single digit reports more common. Studies of “hotspots” in Germany indicated c. 14% anti-SARS-Cov-2 prevalence[15], whereas a study of c. 760 people in Geneva concluded seroprevalence at the end of April was. c. 5.5%[16]. Although meaningfully higher than numbers confirmed by RT-PCR testing, still a long way short of the 60-70% needed to confer herd immunity.
Beyond antibody testing, an initial study published in Cell analysed the T cell response of Covid-19 patients and found patients mount a CD4+ T cell response against SARS-CoV2[17]. Curiously, analysis of a small number of samples taken before the Covid-19 pandemic (n=20) found 40-60% of patients had some degree of cross-reactive T cells against SARS-CoV2 epitopes. It must be stressed this data should not be over-interpreted as we do not know whether i) this cross-reactive T cell response is sufficient to confer protection ii) what level of T-cells are required for protection iii) whether this small sample is representative of the wider population – further studies are warranted to validate the findings.
[1] https://nextstrain.org/ncov/global?l=clock
[2] https://nextstrain.org/flu/seasonal/h3n2/ha/2y?l=clock
[3] https://www.theatlantic.com/health/archive/2020/05/coronavirus-strains-transmissible/611239/
[4] https://www.fda.gov/media/137773/download
[5] https://medcitynews.com/2020/05/feng-zhangs-sherlock-gets-first-ever-crispr-nod-as-fda-green-lights-covid-19-test-kit/
[6] https://www.medrxiv.org/content/10.1101/2020.04.30.20085613v1
[7] https://www.independent.co.uk/news/world/asia/coronavirus-twice-immune-test-positive-recovery-south-korea-latest-a9472091.html
[8] https://www.medrxiv.org/content/10.1101/2020.04.15.20066407v1.full.pdf
[9] https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance
[10] https://www.gov.uk/government/publications/covid-19-laboratory-evaluations-of-serological-assays
[11]https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/887222/PHE_Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2.pdf
[12] https://www.retailgazette.co.uk/blog/2020/05/uk-regulator-retailers-stop-covid-19-antibody-test-kits/
[13]https://wwwnc.cdc.gov/eid/article/13/10/07-0576_article, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5038413/
[14] https://www.sciencemag.org/news/2020/04/antibody-surveys-suggesting-vast-undercount-coronavirus-infections-may-be-unreliable
[15] https://www.technologyreview.com/2020/04/09/999015/blood-tests-show-15-of-people-are-now-immune-to-covid-19-in-one-town-in-germany/, https://www.land.nrw/sites/default/files/asset/document/zwischenergebnis_covid19_case_study_gangelt_0.pdf
[16] https://www.medrxiv.org/content/10.1101/2020.05.02.20088898v1
[17] https://www.cell.com/cell/pdf/S0092-8674(20)30610-3.pdf
DISCLAIMER
This document and the information contained herein is for information purposes only. The document is provided on a non-reliance basis contains forward looking statements and opinions of the manager, which may be subject to change or revision without notice. Some information contained herein was based on, obtained or derived from data published or prepared by other parties (“Third-Party Information”).
None of Ahren or its affiliates, directors, officers, employees, partners, shareholders or agents (each, an “Ahren Party”) assumes any responsibility for the accuracy of the document or any Third-Party Information. No Ahren Party makes any representation or warranty, express or implied, as to the accuracy or completeness of this document or any Third-Party Information and no Ahren Party shall have any liability to the recipient or any other person relating to or resulting from the use of or reliance on any such information contained herein or any errors therein or omissions therefrom. No warranty, express or implied, or assurance is given by the manager or any of its directors, officers, members or employees.
Nothing contained in this document may be relied upon as a guarantee, promise, assurance or a representation as to the future. Except as otherwise indicated, the information provided in this Investor Letter is based on matters as they exist as of the date listed on the cover and not as of any future date, and will not be updated or otherwise revised to reflect information that subsequently becomes available or circumstances existing or changes occurring after the date hereof. The views expressed in this Investor Letter are subject to change based on market and other conditions. In considering the performance information contained herein, prospective investors should bear in mind that past, forecasted or targeted performance is not necessarily indicative of future results, and there can be no assurance that comparable results will be achieved.
Neither Ahren nor any of its affiliates makes any representation or warranty, express or implied, as to the accuracy or completeness of the information contained herein, and nothing contained herein should be relied upon as a promise or representation as to past or future performance of Ahren or the fund or any other entity.
The recipient acknowledges that, to the maximum extent permitted by law, each of Ahren and its related parties or affiliates disclaims all liability to the recipient or to any other person for any expense, cost, loss or damage of any kind including direct, indirect or consequential loss or damage (however caused, including by negligence) incurred by any person arising from or relating to any information included or omitted from this Investor Letter, whether by reason of such information being inaccurate or incomplete or for any other reason. This Investor Letter does not constitute and should not be considered as any form of financial opinion or recommendation. The recipient should conduct its own inquiries as to the adequacy, accuracy, completeness and reliability of any information, whether such information is contained in this Investor Letter or not, relating to Ahren or the fund.
No action should be taken by any recipient which, in the light of the information herein, would render such action illegal or violate any applicable laws or regulations. Neither the manager nor any of its directors, officers, members or employees accepts any liability whatsoever for its accuracy or any action taken by a recipient which is illegal or in violation of any applicable law or regulation as a result of receiving this document and its contents.
The Site cannot and does not contain medical or health related advice. The medical or health information is provided for general informational and educational purposes only and is not a substitute for professional advice. Accordingly, before taking any actions based upon such information, we encourage you to consult with the appropriate professionals. We do not provide any kind of medical or health advice.
The Site does not offer, and should not be construed in any way as offering legal, regulatory or tax advice. The information is provided for general informational purposes only and is not a substitute for professional legal or tax advice.
THE USE OR RELIANCE OF ANY INFORMATION CONTAINED ON THIS SITE IS SOLELY AT YOUR OWN RISK.
 22 December, 2020
Professor Zoubin Ghahramani: Ahren Podcast Series – A Glimpse into the Future with Ahren’s Science Partners
22 December, 2020
Professor Zoubin Ghahramani: Ahren Podcast Series – A Glimpse into the Future with Ahren’s Science Partners
 18 December, 2020
Ahren Innovation Capital’s Annual Symposium – Science Partner Panel Highlights 2019
18 December, 2020
Ahren Innovation Capital’s Annual Symposium – Science Partner Panel Highlights 2019