6 April, 2020
In a matter of weeks, the international scientific effort to tackle Covid-19 has showcased the best of human innovation and creativity. Below we have briefly summarised positive developments in therapeutics, testing, and other efforts in the attempt to combat Covid-19.
We must mention, however, that given the urgency, it is possible that rushing potential vaccines, antivirals and antibody therapies (understandably and positively) into clinics might be done without the opportunity for due process. If this happens, they may not pass safety tests in clinical trials, for example, if they create Antibody Dependent Enhancement (“ADE”) of the form we describe below. It is important for the scientific community to try to under-promise and over-deliver: while it would be excellent if a quick solution were found, there is a high probability that there will be failures before success. This is the nature of science and scientific progress; we hope that the global community will recognise this and not be over-hopeful and then despair unwarranted. We simply need to keep going.
ACE2 Receptor – A receptor found on human lung alveolar epithelial cells (as well as other cell types) that binds SARS-CoV-2 virus and facilitates infection
Antibody/Immunoglobulin – Natural proteins produced by the immune system that are designed to bind and neutralise invading pathogens
Coronavirus – A group of related viruses that cause diseases in mammals and birds
Covid-19 – The disease caused by the SARS-CoV-2 Virus
Pathogen – A bacteria, virus or other microorganism that can cause disease
Plasma – Liquid part of the blood that is cell free but is rich in antibodies and other proteins
SARS – The disease caused by the SARS-CoV virus in 2002-2003
SARS-CoV-2 – The virus that causes Covid-19
SARS-CoV The virus responsible for the 2003-2003 SARS outbreak
Spike Protein – A protein found on the outside of SARS-CoV-2 viral particles that binds to ACE2 receptors on human cells to facilitate infection
Therapeutics to address Covid-19 can take various approaches and are summarised in Table 1.
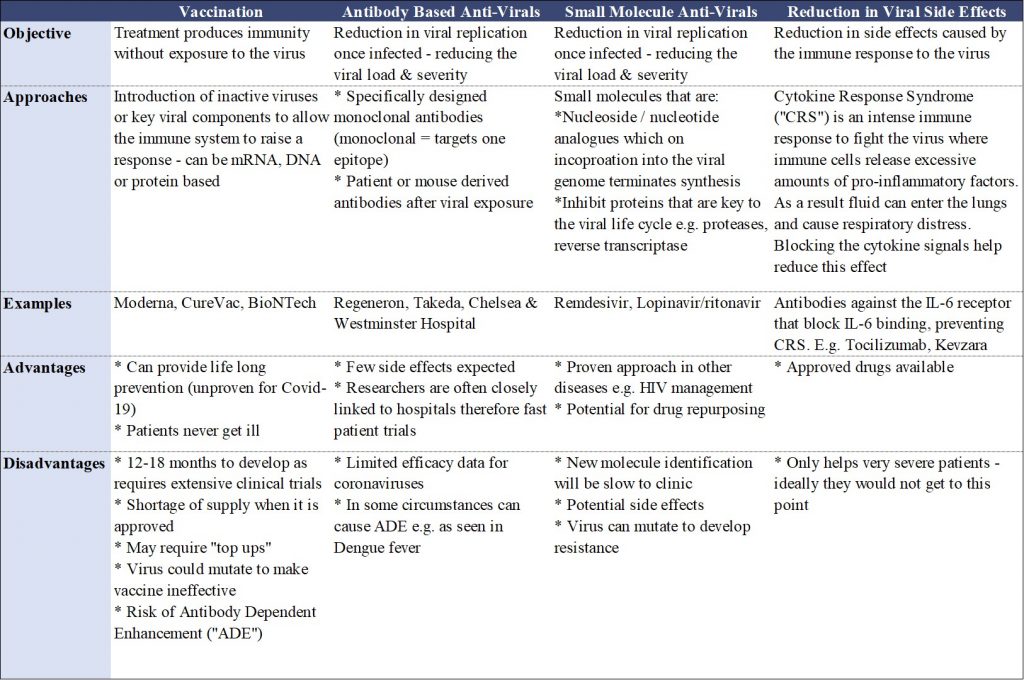
Table 1. A summary of the current therapeutic approaches being undertaken by researchers to address Covid-19.
Vaccination involves exposure to an inactive virus or viral proteins, stimulating the immune system to raise a response. When the patient is then exposed to the pathogen, the primed immune system quickly raises a strong response to overcome the pathogen (Figure 1). If effective, patients never become ill and in some instances the protection can be lifelong.
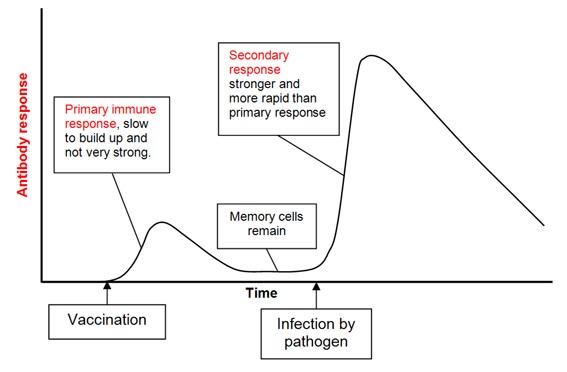
Figure 1. Theory of how vaccination stimulates an immune response, so that on exposure to the pathogen (virus) the immune response is strong and rapid. Source: BioKnowledgy Presentation.
Unfortunately, it is likely to take at least 12-18 months to develop a vaccine, with significant challenges ahead. The virus may mutate rendering the vaccination ineffective, immunity may be short lived and require annual, or even more frequent, top-ups and there will be an inevitable shortage of supply after regulatory approval. Not all diseases have effective vaccines – it is notable that many common colds are coronaviruses which have notoriously evaded vaccination. Finally, there is a risk the vaccine may make the disease worse rather than better, through Antibody Dependent Enhancement, (“ADE”). ADE occurs when non-neutralising antibodies facilitate the uptake of viral particles into immune cells called macrophages. The virus can then replicate further in a new host cell and be carried through the body, making the disease more severe. This phenomenon is rare but has been seen in Dengue Fever, HIV and preliminarily with the closely related SARS-CoV virus that caused the 2003 SARS outbreak (1).
However, many leading scientists and biotech companies have not been discouraged! As of 26th March, there were 54 independent SARS-CoV-2 vaccines under development registered with the World Health Organisation (“WHO”) (2). On the 16th March, vaccines from the first two companies entered the clinic – Massachusetts based Moderna and a Chinese company called CanSino.
Anti-viral therapies are either:
Both approaches inhibit viral replication, reducing the viral “load” during infection. Fortunately, an abundance of anti-virals are on the market and used successfully to manage viral diseases such as HIV. Trials are underway to test small molecule anti-viral drugs including Remdesivir which was developed to treat Ebola (nucleotide analogue), and Lopinavir & Ritonavir (viral protease inhibitors) which are approved to treat HIV. The WHO has launched a centralised effort to review the clinical efficacy of these drugs along with Chloroquine – an antimalarial molecule that has received a lot of press attention, although there is not yet definitive supporting evidence of efficacy.
Antibodies are proteins secreted by B-Cells of the immune system that specifically bind viral and bacterial “antigens” with a complementary shape to the antibody – analogous to a key fitting into a lock (Figure 2). This binding of the antibody can “neutralise” the invading bacteria or virus by disrupting their normal function, as well as alerting host immune cells to engulf and destroy the pathogen. For example, SARS CoV (virus behind 2003 SARS outbreak) and SARS CoV-2 (virus causing the ongoing pandemic) enter cells when the viral “Spike” protein binds to ACE2 receptors expressed on epithelial cells. The schematic below demonstrates how a neutralising antibody can bind the Spike protein and prevent the virus engaging host ACE2 receptors.
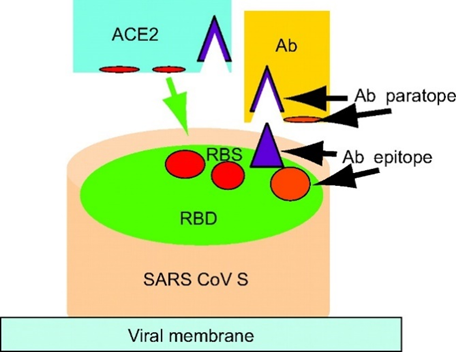
Figure 2. Schematic explaining how the SARS CoV Spike Protein on the viral membrane, which attempts to bind ACE2 receptors on host cells in order to gain entry, is “neutralised” by an antibody (Ab) (Source: Zhu et al., PNAS 2007).
Antibody based therapies tend to be relatively safe, but as antibodies need to be developed that specifically bind SARS-CoV-2, there are no existing products on the market. Numerous companies and consortiums are developing neutralising antibodies, by isolating antibodies for example from mice inoculated with SARS-CoV-2 or convalescent plasma, as described below. Regeneron has been moving notably swiftly and is aiming to enter the clinic with an antibody cocktail this summer.
A second approach to obtaining neutralising antibodies is directly from the plasma of recently recovered, confirmed Covid-19 patients (“Convalescent Plasma”). The plasma will contain a high concentration of antibodies specifically against SARS-CoV-2 and is a method of passively boosting the immune response. This approach has shown efficacy against polio, measles, mumps, the 1918 Flu Pandemic as well as SARS and MERS (3). A very small study in China (n=5) demonstrated potential efficacy in Covid-19 patients (4). On the 26th March the FDA approved use of convalescent plasma for critically ill Covid-19 patients. In the US a consortium of hospitals and institutions are collaborating, using expertise and infrastructure already established for blood donation.
Finally, for the patients that are critically ill there are therapies that reduce the damage caused by a severe immune response which can be fatal. Cytokines are small proteins that coordinate the body’s response to infection and trigger inflammation. If this goes into overdrive (a “cytokine storm”) it can cause severe inflammation, leading to fluid entering the lungs and acute respiratory distress. One of the key cytokines involved is Interleukin-6 (“IL-6”). Two monoclonal antibodies (Tocilizumab and Kevzara – developed using the technology originally pioneered by Ahren Science Partner Sir Greg Winter) bind and block the receptor for IL-6 (“IL-6R”), thereby reducing the effect of the cytokines. The antibodies have shown previous efficacy in other conditions and are being trialled to relieve respiratory distress in critical Covid-19 patients.
There are two categories of testing for Covid-19. The first is testing for the presence of viral RNA using a Reverse Transcription Polymerase Chain Reaction (“RT- PCR”), a basic laboratory technique which detects specific RNA sequences found in the SARS-CoV-2 viral genome. The test is only useful during an ongoing infection, and not useful after the virus has been cleared and the patient has recovered. Many universities, hospitals and companies have risen to the challenge to provide testing. As of 31st March 2020, 20 diagnostic tests had been granted Emergency Use Authorisation by the FDA (5) including Roche’s rapid diagnostic which produces results in less than four hours with 400,000 tests shipped per week and AbbVie who were aiming to ship one million tests per week by the end of March.
The second type of testing is for assumed immunity from the virus – i.e. you have been exposed to the virus and generated antibodies against it so are unlikely to fall ill again or infect others. This requires a blood sample which is screened for antibodies that bind core viral proteins. The technology is established – and known as an ELISA test (Figure 3), which is used commonly for example in pregnancy testing. A SARS-CoV-2 protein is immobilised on a surface and if the blood contains antibodies against SARS-CoV-2 they bind to the protein. After washing, the bound antibodies remain and are readily detected using a secondary antibody. Detection is displayed as a colour change (analogous to a coloured line appearing on a pregnancy test). Tests developed by Duke-NUS Medical School were used in Singapore and multiple companies have developed prototypes which are being tested by the FDA and UK regulatory bodies. The immediate challenge is ensuring the tests are sufficiently accurate: i.e. have high sensitivity (detects everyone who have had the disease) and specificity (doesn’t accidentally ascribe a false positive to those who have not had the disease). Beyond this, there are three other challenges: (a) while this tests for antibodies generated against the disease, we do not have evidence that this in fact confers immunity (although encouragingly, there is evidence it did so for a period in the case of SARS and MERS) (6); (b) we do not currently have evidence that having antibodies against the virus does not mean that one cannot remain infectious to others; (c) the virus has the capacity to mutate, meaning we may not be able to rely fully on such tests to enable self-isolation to stop. However, in theory this should be less of an issue as the virus will likely elicit many different antibodies against both mutated and unmutated regions.
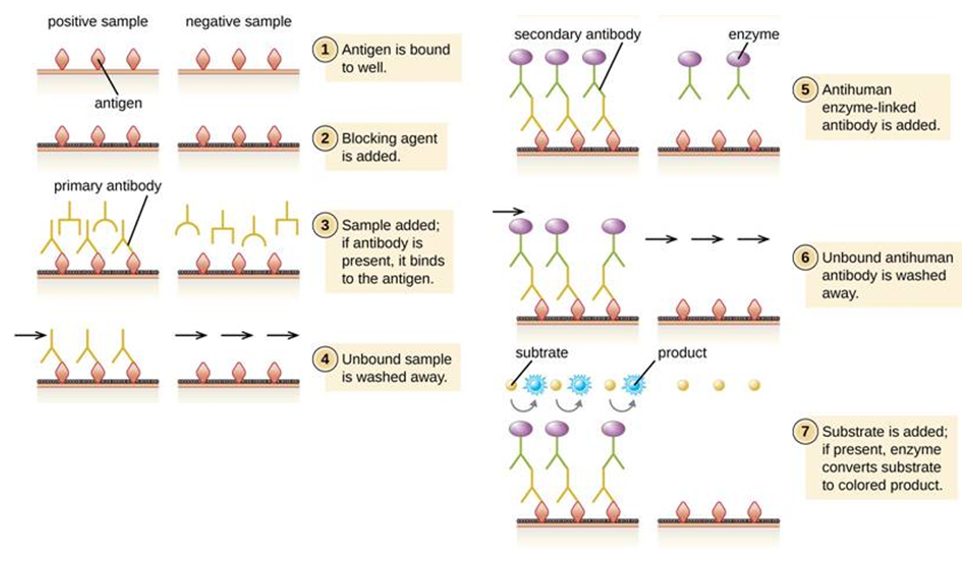
Figure 3. Schematic for how an ELISA (enzyme-linked immunosorbent assay) works, that detects serum antibodies against viral antigens (proteins). The antigen will be an immobilised SARS-CoV-2 protein, likely the Spike protein. Source: Lumen Learning.
Every day more studies are being published explaining the biology of the virus such as the viral structure (7), how the virus binds and enters cells (via a receptor called ACE2) (8) and its survival time on surfaces (9), to name a few. Machine Learning and AI is also contributing. For example, DeepMind is using its AlphaFold data system (an algorithm that predicts protein structure from genomic data) to predict the structures of several under-studied proteins associated with SARS-CoV-2 in order to aid in viral function research and therapeutic development (10) and the J-Clinic at MIT has launched a consortium to support the Covid-19 response. Supportive capital is being deployed by individuals and institutions including the Bill and Melinda Gates Foundation, the Wellcome Trust and Mastercard who in collaboration committed $125M to aid therapeutic discovery; and the UK’s Medical Research Council which announced £20M in research funding for various products. Large pharmaceutical companies are donating drugs including Novartis who committed £130M of Chloroquine. Technology may also contribute in managing the pandemic including websites and apps to collect populational data to aid hospital planning and epidemiological studies (for example the “C-19 Symptom Tracker App”).
1. Yang et al., Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. PNAS 2005
2. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov
3. https://www.nature.com/articles/d41586-020-00895-8
4. Shen et al, JAMA Network March 2020
5. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations-medical-devices#covid19ivd
6. Wu et al., Emerging Infectious Diseases 2007. Payne et al., Emerging Infectious Diseases 2016
7. Zhang et al., Science 2020
8. Hoffmann et al., Cell 2020
9. van Doremalen et al., NEJM 2020
10. https://deepmind.com/research/open-source/computational-predictions-of-protein-structures-associated-with-COVID-19
DISCLAIMER
This document and the information contained herein is for information purposes only. The document is provided on a non-reliance basis contains forward looking statements and opinions of the manager, which may be subject to change or revision without notice. Some information contained herein was based on, obtained or derived from data published or prepared by other parties (“Third-Party Information”).
None of Ahren or its affiliates, directors, officers, employees, partners, shareholders or agents (each, an “Ahren Party”) assumes any responsibility for the accuracy of the document or any Third-Party Information. No Ahren Party makes any representation or warranty, express or implied, as to the accuracy or completeness of this document or any Third-Party Information and no Ahren Party shall have any liability to the recipient or any other person relating to or resulting from the use of or reliance on any such information contained herein or any errors therein or omissions therefrom. No warranty, express or implied, or assurance is given by the manager or any of its directors, officers, members or employees.
Nothing contained in this document may be relied upon as a guarantee, promise, assurance or a representation as to the future. Except as otherwise indicated, the information provided in this Investor Letter is based on matters as they exist as of the date listed on the cover and not as of any future date, and will not be updated or otherwise revised to reflect information that subsequently becomes available or circumstances existing or changes occurring after the date hereof. The views expressed in this Investor Letter are subject to change based on market and other conditions. In considering the performance information contained herein, prospective investors should bear in mind that past, forecasted or targeted performance is not necessarily indicative of future results, and there can be no assurance that comparable results will be achieved.
Neither Ahren nor any of its affiliates makes any representation or warranty, express or implied, as to the accuracy or completeness of the information contained herein, and nothing contained herein should be relied upon as a promise or representation as to past or future performance of Ahren or the fund or any other entity.
The recipient acknowledges that, to the maximum extent permitted by law, each of Ahren and its related parties or affiliates disclaims all liability to the recipient or to any other person for any expense, cost, loss or damage of any kind including direct, indirect or consequential loss or damage (however caused, including by negligence) incurred by any person arising from or relating to any information included or omitted from this Investor Letter, whether by reason of such information being inaccurate or incomplete or for any other reason. This Investor Letter does not constitute and should not be considered as any form of financial opinion or recommendation. The recipient should conduct its own inquiries as to the adequacy, accuracy, completeness and reliability of any information, whether such information is contained in this Investor Letter or not, relating to Ahren or the fund.
No action should be taken by any recipient which, in the light of the information herein, would render such action illegal or violate any applicable laws or regulations. Neither the manager nor any of its directors, officers, members or employees accepts any liability whatsoever for its accuracy or any action taken by a recipient which is illegal or in violation of any applicable law or regulation as a result of receiving this document and its contents.
The Site cannot and does not contain medical or health related advice. The medical or health information is provided for general informational and educational purposes only and is not a substitute for professional advice. Accordingly, before taking any actions based upon such information, we encourage you to consult with the appropriate professionals. We do not provide any kind of medical or health advice.
The Site does not offer, and should not be construed in any way as offering legal, regulatory or tax advice. The information is provided for general informational purposes only and is not a substitute for professional legal or tax advice.
THE USE OR RELIANCE OF ANY INFORMATION CONTAINED ON THIS SITE IS SOLELY AT YOUR OWN RISK.
 22 December, 2020
Professor Zoubin Ghahramani: Ahren Podcast Series – A Glimpse into the Future with Ahren’s Science Partners
22 December, 2020
Professor Zoubin Ghahramani: Ahren Podcast Series – A Glimpse into the Future with Ahren’s Science Partners
 18 December, 2020
Ahren Innovation Capital’s Annual Symposium – Science Partner Panel Highlights 2019
18 December, 2020
Ahren Innovation Capital’s Annual Symposium – Science Partner Panel Highlights 2019